

Formulated for POWERFUL CLEANSING and BIOBURDEN REMOVAL



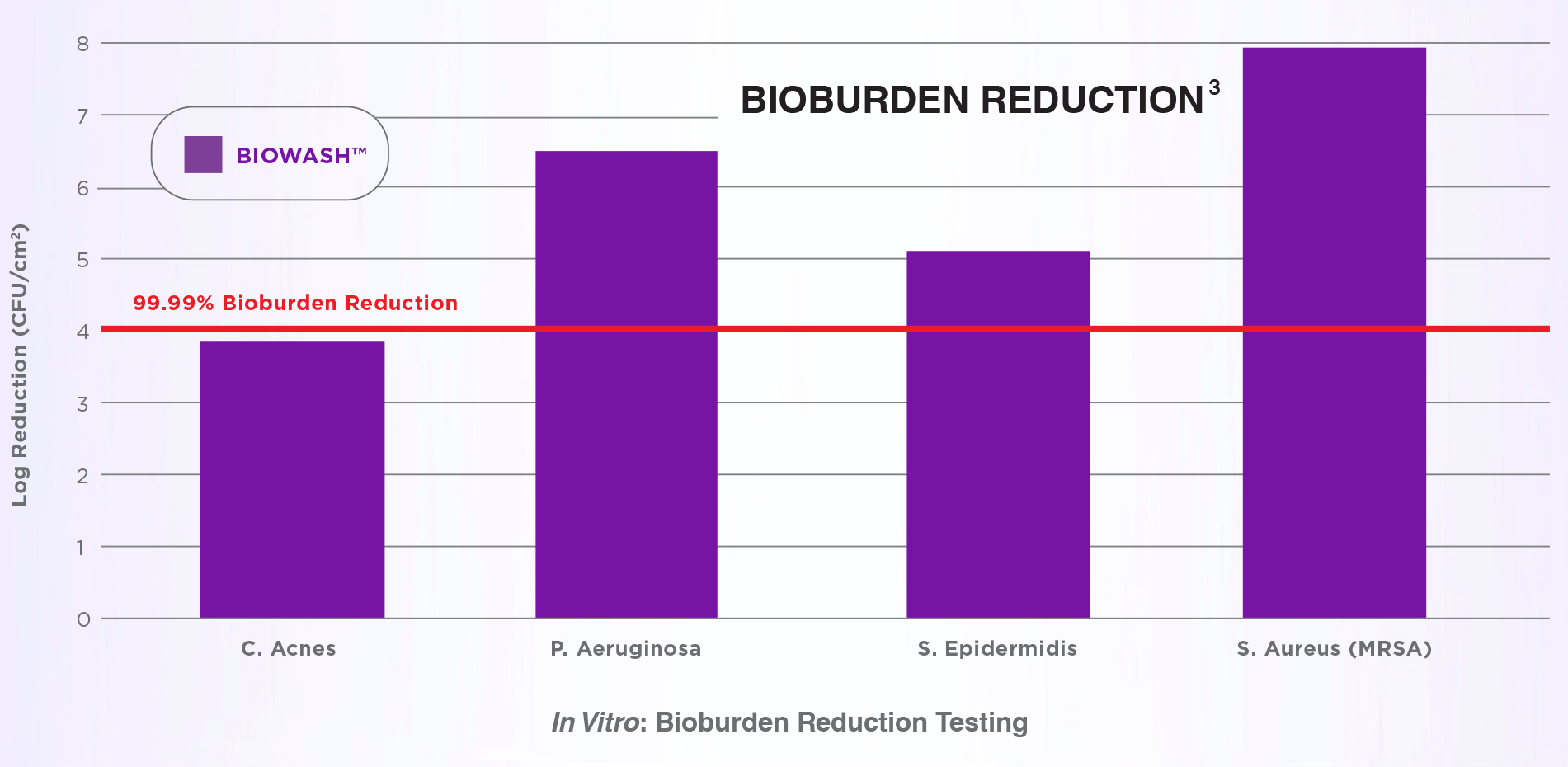
Testing of BIOWASH™ was conducted against pathogens which commonly cause infections. Compared to irrigants requiring immediate rinsing, residual BIOWASH™ product can safely remain in the wound. In Vitro testing of BioWASH shows a reduction in bioburden contamination in the packaging.
REFERENCES
1. Documents: NMS-40027 Rev C
2. BIOWASH™ meets ISO 10993 biocompatibility requirements for ≤ 24 hours contact on breached or compromised surfaces. Data on file.
3. Data on file, n=3
NOTE: In Vitro results have not been shown to definitively correlate with clinical outcomes.
MKT128
